The COVID-19 vaccine has been approved for children aged 12-17, and the government is preparing to extend the vaccination program to those aged 5-11. This initiative not only helps protect children from the virus and boosts their immunity but also enables them to resume their normal activities more safely. So, what are the recommendations from the WHO regarding COVID-19 vaccination for children? Let’s find out in the article below.
1. Possible Side Effects After Children Receive the COVID-19 Vaccine
According to the Health and Life newspaper, side effects may appear or not in some children depending on their immunity and physical condition. However, most side effects of the COVID-19 vaccine are typically mild to moderate and will subside within a few days.
Some possible side effects that may occur in children include:
- Pain, redness, or swelling at the injection site or throughout the body
- Fatigue, headache, muscle pain, fever, nausea, etc.
- In some cases, children may experience swollen lymph nodes in the armpit or neck or develop a red, itchy rash on the injected arm, known as “COVID arm.”
Clinical trials have shown that children tend to experience a higher fever than adults.
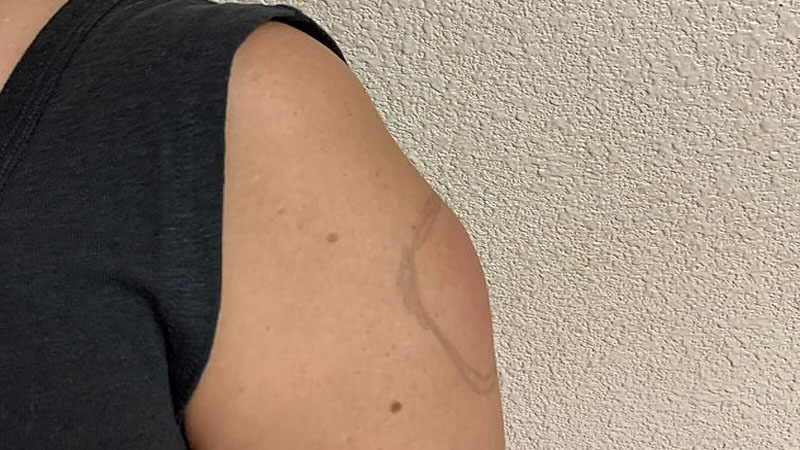 COVID arm
COVID arm
In addition to these reactions, there are some rare side effects that mainly affect males, including myocarditis and pericarditis, with symptoms such as shortness of breath, chest pain, and rapid heartbeat.
2. How to Care for Children After Receiving the COVID-19 Vaccine
For safety reasons, parents should educate their children about the vaccination and inform the doctor about any allergies the child may have. It is not recommended to give pain relievers to children before vaccination.
After the vaccination, parents should remain with their children for 15-30 minutes at the vaccination center to observe for any immediate adverse reactions, as medical staff will be on hand to provide assistance if needed.
Additionally, consult a doctor before giving your child any medication, including over-the-counter drugs like panadol, ibuprofen, or naprosyn, to alleviate pain or fever caused by side effects. For medications that your child takes daily, continue to administer them as usual.
 Caring for children after the COVID-19 vaccination
Caring for children after the COVID-19 vaccination
To reduce pain or swelling at the injection site without medication, you can:
- Apply a clean, cold compress to the area or encourage gentle arm movement.
- To ease discomfort from fever, ensure your child stays hydrated, eats nutritious foods, and wears comfortable, loose-fitting clothing.
If your child experiences severe symptoms such as a fever above 38°C, shortness of breath, rapid heartbeat, anaphylaxis, or worsening symptoms at the injection site after 24 hours, contact your doctor or nearest medical center immediately for advice and treatment.
3. WHO Recommendations for COVID-19 Vaccines for Children
There is a misconception that children do not need the COVID-19 vaccine because they are less likely to become infected. However, children who contract the virus are still at risk of experiencing severe side effects, similar to adults, even if they are asymptomatic or have mild symptoms when detected.
Therefore, the World Health Organization (WHO) recommended in July 2021 that children aged 12-17 receive the COVID-19 vaccine.
Vietnam’s Ministry of Health has also recently requested that local authorities prepare to administer the vaccine to children aged 5-11, starting from October 2021.
For children aged 5 to under 12: Each tray contains 195 vials, with 10 doses per vial; each box contains 10 vials, with 10 doses per vial. The vaccine is manufactured by Pfizer Manufacturing Belgium NV (Belgium), BioNTech Manufacturing GmbH (Germany), Pharmacia and Upjohn Company LLC (USA), and Hospira Incorporated (USA).
Decision 457 takes effect from today, March 1st, revoking Decision No. 4035/QD-BYT dated August 21st, 2021, of the Minister of Health.
 WHO recommendations for COVID-19 vaccines for children
WHO recommendations for COVID-19 vaccines for children
The WHO’s group of experts (SAGE) concluded that the Pfizer-BioNTech vaccine is approved for individuals aged 16 and older and authorized for emergency use for those aged 12-15.
Furthermore, the US Centers for Disease Control and Prevention (CDC) recommends that all children over 12 years old should receive the COVID-19 vaccine to help curb the pandemic. The vaccine dosage for this age group remains the same as for adults, and the interval between the two doses is three weeks.
For further information:
The above information provides essential details about the COVID-19 vaccine for children. Stay tuned for more updates!
Sources: World Health Organization (WHO), Health and Life newspaper