What is Purple Litmus? Advantages of Blue Litmus Compared to Other pH Indicators
Purple litmus, also known as litmus paper, is a paper that has been impregnated with an ethanol solution or water containing a colorant. It is derived from the roots of lichens called Roccella and Dendrographa. Originally, the paper was purple in color and was used for chemistry experiments and pH measurements. After use, the litmus paper will change to a different color.
The main advantage of blue litmus, compared to other pH indicators, is its fast results, making it particularly suitable for experiments. Additionally, litmus paper can be used to distinguish gases. Therefore, purple litmus is an indispensable tool in current experiments or laboratory settings.

Classification of Purple Litmus
Purple litmus paper is classified into two main types: red litmus paper and blue litmus paper.
- Red litmus paper: Made by treating plain paper with a dye soaked in a dilute solution of sulfuric acid. The paper is then dried by direct contact with the air.
- Blue litmus paper: When dipped in a solution, if the paper turns red, the solution is acidic; if the paper does not change color, the solution is neutral. Blue litmus is used to identify acids and vinegars.
Additionally, litmus paper can be further categorized as moist purple litmus and dry purple litmus. To differentiate between the two, one only needs to expose them to ammonia gas. Dry purple litmus will not change color, while moist purple litmus will turn blue.
How Does Purple Litmus Change Color?
Purple litmus can change color depending on whether the solution it comes into contact with is acidic, basic, or neutral:
- Purple litmus turns red when exposed to an acidic solution.
- Purple litmus turns blue when exposed to a basic solution.
- Blue litmus does not change color when exposed to a neutral solution.

Applications of Purple Litmus
Purple litmus has numerous applications in science and daily life, such as distinguishing chemicals, measuring pH, and testing amniotic fluid.
Used to Distinguish Chemical Solutions
A small piece of litmus paper can be used to identify whether a solution is basic or acidic.
- When purple litmus comes into contact with acids (e.g., HCL, H2SO4), red litmus turns red.
- When blue litmus reacts with bases (e.g., NaOH, KOH), litmus turns greenish-blue.
- Blue litmus does not change color when the solution is neutral.

Measure pH with Red Litmus Paper
Litmus paper can provide a quick measurement of pH. However, the results obtained from purple litmus are only approximate and not 100% accurate. For more accurate measurements, a pH meter should be used.
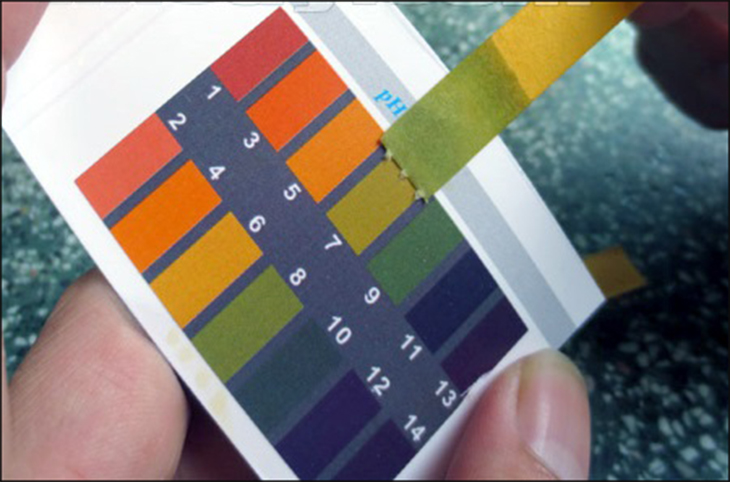
To conduct a quick measurement using purple litmus, tear off a piece of litmus paper and dip it in water, then compare the color with the accompanying chart.
- If the pH is from 1 to 7, the environment is acidic.
- If the pH is from 7 to 14, the environment is basic.
- If the litmus paper shows a pH of 7, the medium is neutral.
Purple litmus can also be used to test for amniotic fluid leakage in pregnant women during the final stage of pregnancy. This test helps determine the well-being of the fetus inside the mother’s womb and enables timely intervention if needed.

Where to Buy Purple Litmus?
We hope this article has provided you with a better understanding of purple litmus. If you have any questions, please feel free to leave a comment below.