At the recently concluded EuroPCR 2025, a leading cardiovascular conference held in Paris, a team of medical technology experts from the United States presented the three-year clinical study results of the DynamX Bioadaptor, a next-generation bioresorbable vascular scaffold. This announcement comes on the heels of Elixir Medical being recognized by Fast Company as one of the world’s top 10 most innovative medical device companies of 2025.
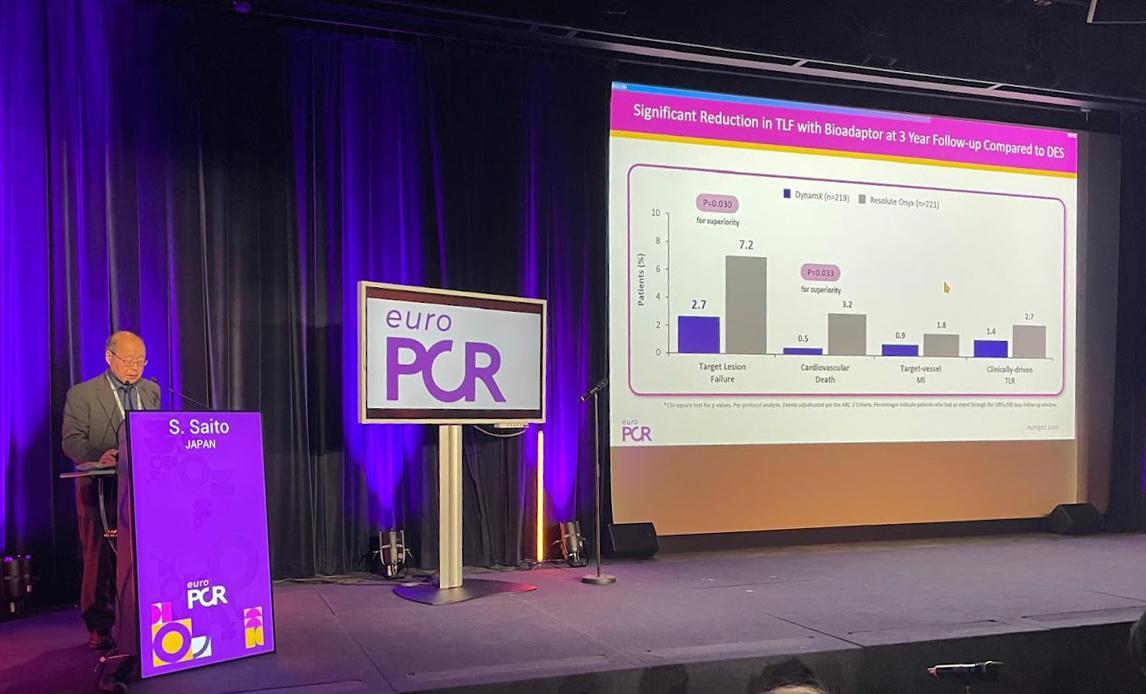
Speaking at EuroPCR 2025, Professor Shigeru Saito highlighted the DynamX’s unique advantages over drug-eluting stents (DES) in the treatment of coronary artery disease.
Professor Saito, Director of Cardiology and Arterial Research at Shonan Kamakura General Hospital in Japan, presented the findings from the BIOADAPTOR RCT trial involving 445 patients across 34 centers in Japan, Europe, and New Zealand. The results demonstrated the superiority of the DynamX Bioadaptor over traditional drug-eluting stents. After three years, the BIOADAPTOR RCT trial reported a target lesion failure (TLF) rate of only 2.7% in the DynamX Bioadaptor group, significantly lower than the 7.2% observed in the DES group. This translates to a 63% reduction, indicating the long-term stability and effectiveness of the DynamX Bioadaptor.
“The difference is evident not only in the overall numbers but also in the individual components of TLF. Specifically, cardiovascular death was reduced by 84% in the DynamX group compared to the DES group (0.5% vs. 3.2%). Target vessel myocardial infarction was halved (0.9% vs. 1.8%), while the rate of ischemia-driven target lesion revascularization was 1.4% with DynamX, compared to 2.7% with drug-eluting stents.” – Professor Saito explained.
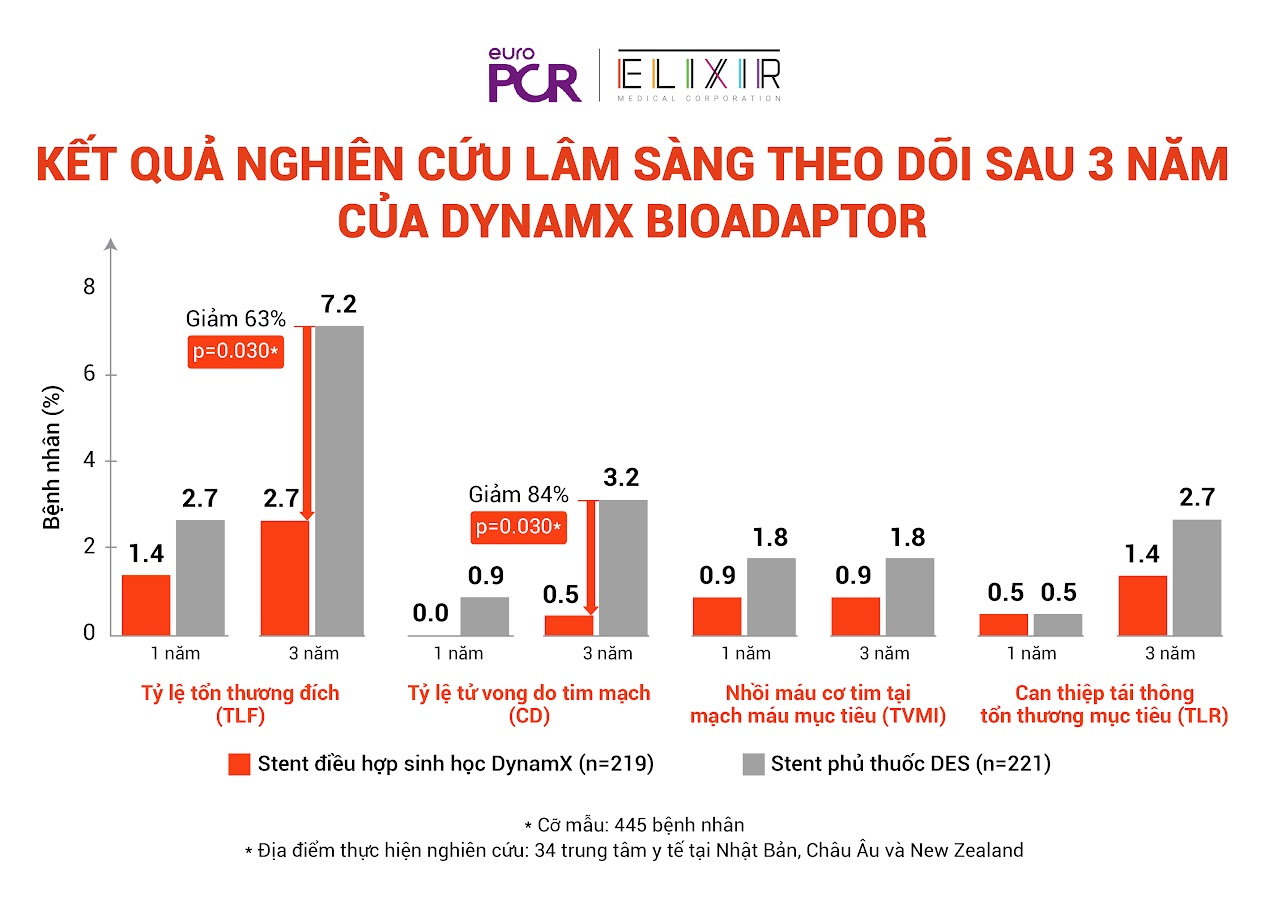
The DynamX Bioadaptor showed significant improvements across all three TLF criteria: cardiovascular death, myocardial infarction, and revascularization, when compared to drug-eluting stents (DES)
Associate Professor Dr. Huynh Van Thuong, former Deputy Director of Khanh Hoa Provincial General Hospital, elaborated on the unique mechanism of this bioresorbable scaffold: “Bioresorbable scaffolds have special joints that detach after six months, restoring the vessel wall’s mobility. With this feature, the DynamX Bioadaptor can support the recovery of the blood vessel’s physiological function, reduce atherosclerotic plaque, and significantly lower the risk of long-term complications. This is a revolutionary step forward in the treatment of coronary artery disease, offering new hope to patients with stable and long-term treatment outcomes.”
Dr. Ly Ich Trung, Head of Cardiovascular Intervention at Cho Ray Hospital, commented: “The three-year results of the DynamX Bioadaptor demonstrate a 63% lower risk of target lesion failure compared to drug-eluting stents (DES) after three years. The DynamX’s unique bio-adaptive mechanism is particularly suitable for young patients who require a flexible, low-complication treatment solution with long-term effectiveness, thereby improving their quality of life.”
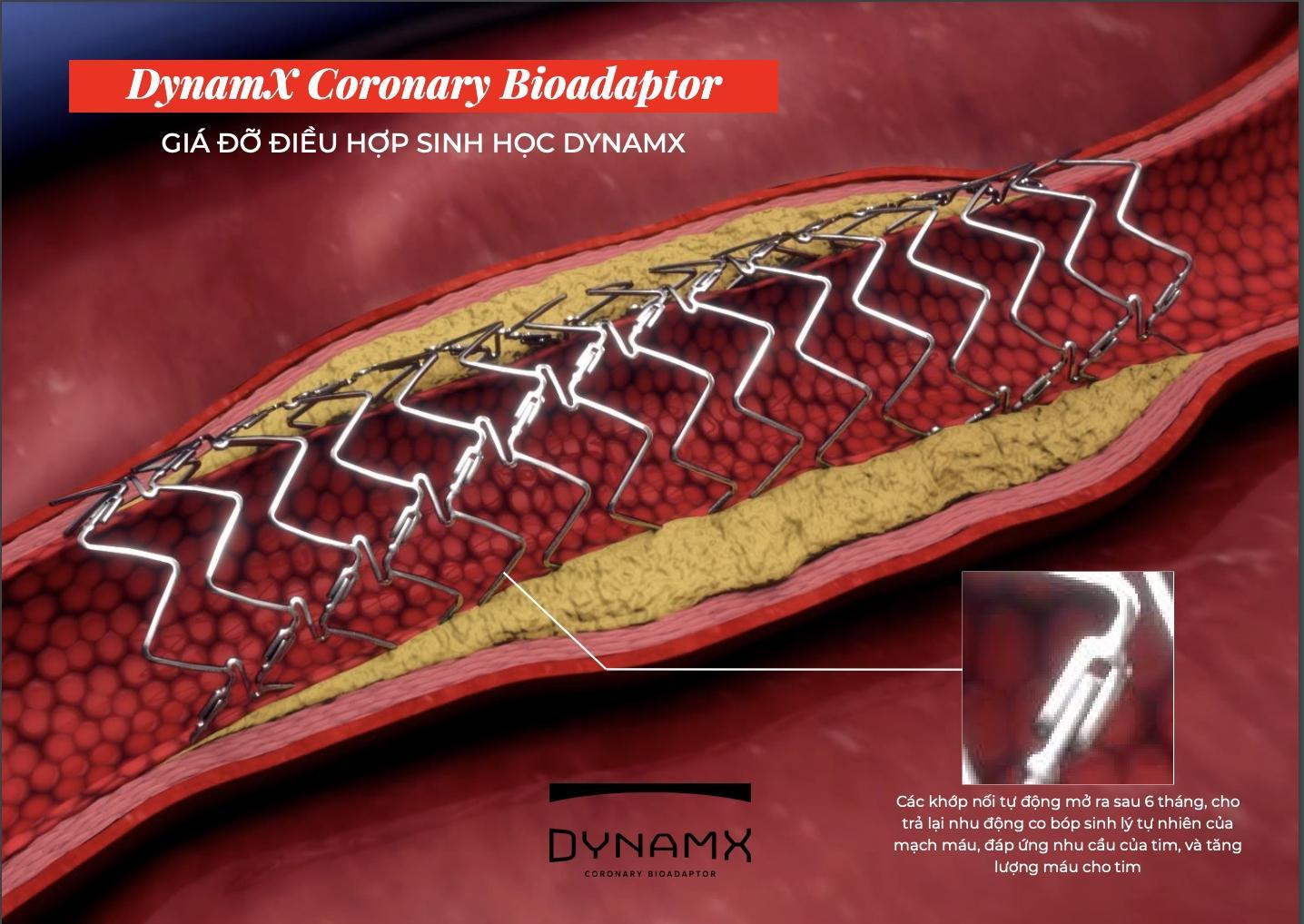
The DynamX Bioadaptor’s unique structure with self-detaching joints “frees” the blood vessel after intervention, offering long-term stability.
In Vietnam, the DynamX Bioadaptor technology is being adopted by numerous hospitals nationwide, including central institutions such as Cho Ray Hospital, Ho Chi Minh City University Medical Hospital, and Ho Chi Minh City Institute of Heart Disease, as well as large provincial hospitals like Khanh Hoa, Tra Vinh, and Can Tho Central General Hospitals.
Pharmacist Nguyen Quoc Bao, representative of Elixir Medical Vietnam, shared: “To date, approximately 40 hospitals across Vietnam have utilized the DynamX technology in treating coronary artery disease. Notably, despite being a significantly different device from drug-eluting stents and recognized by the FDA as a breakthrough technology in 2024, the bidding price for this device in Vietnam is considerably lower than in many other countries, providing a reasonable and accessible solution for patients. Furthermore, the DynamX Bioadaptor has been approved by the Ministry of Health and is reimbursable by health insurance.”
|
Elixir Medical is a medical technology company headquartered in Milpitas, California, USA, specializing in the research and development of advanced medical devices for the treatment of cardiovascular and peripheral vascular diseases. The company focuses on solutions that restore physiological function rather than solely relying on mechanical intervention, thereby offering patients enhanced long-term safety and effectiveness. |